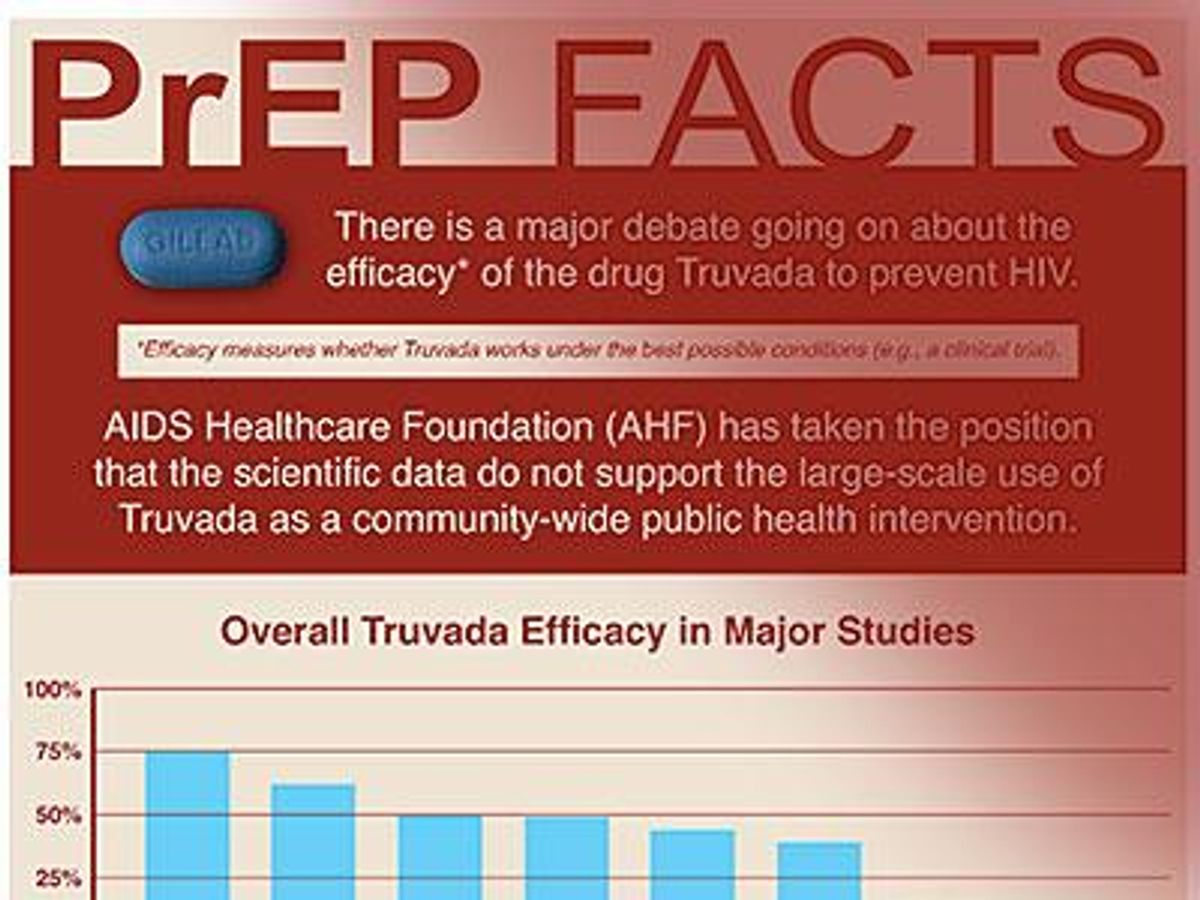
Escalating the war of words over PrEP, The AIDS Healthcare Foundation has announced a major new print advertising campaign denigrating the once-a-day HIV prevention option.
The new campaign began appearing in print publications in California and Florida August 17 and claims PrEP is not nearly as effective as the federal government says it is because of low adherence rates. Without a doubt, adherence to daily medication is a problem — for those living with HIV and those who are HIV-negative — and there are a lot of reasons for that.
But what the campaign does not discuss, or disclose, is that when taken daily, Truvada is incredibly effective in preventing new infections.
AHF, however, wants readers to believe that PrEP is only as effective as the baseline results from various studies. A baseling finding combines all the results together to come up with an overall efficacy result. But those studies found substantial differences between participants with detectable levels of the drug in their blood and those who did not. But science requires that we combine all the groups — adherent and nonadherent — for one overall efficacy rate. AHF would like folks to believe those low numbers are the topline of efficacy, when in fact they are the bottom line of efficacy.
To compare, AHF loves to promote condoms — and more power to them as they can be effective.
However, a study by Centers for Disease Control and Prevention researcher Dawn Smith found that when not used correctly and consistently every time for anal sex had no net protective effect over not using condoms. But using condoms every time, correctly and consistently during anal sex had a 70 percent reduction in HIV transmission. So, using AHF's logic, encouraging condom use is futile since only one in six men who have sex with men report using condoms each and every time. Therefore, adherence to condoms results poor efficacy in HIV transmission prevention.
Sounds pretty ridiculous, doesn't it? Yet that is exactly what AHF is doing when it comes to PrEP. The National Institute of Health says the intervention is up to 99 percent effective when taken daily.
The FDA approved Truvada for this intervention in July 2012, based on those very same studies AHF would like you to believe say the drug doesn't work. And the CDC earlier this year released new clinical guidance for PrEP which also cites the efficacy of everyday use of PrEP in preventing infection — not the AHF's abuse of the science.
Since FDA has regulatory authority over advertising of medical devices and drugs, perhaps it should step up and counter this misinformation. Or, perhaps Gilead, the maker of Truvada, can break its silence and challenge the misinformation under a June 2014 draft FDA guidance for addressing third party, unaffiliated claims.